JLMDC is a double-blind, peer-reviewed journal published biannually. It serves as a medium for the latest advancement of scientific knowledge in all the branches of Medicine and its allied sciences and the publication of scientific investigation in these fields.
OJS user guide for submission of Research Article to the JLMDC
JLMDC follows the ICMJE guidelines “Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly work" in medical journals; available at https://icmje.org/recommendations/
Separate guidelines/checklists for different types of study design are hosted by the Equator Network at https://www.equator-network.org/reporting-guidelines/consort/
Human research Reporting Guidelines and Best Practices: Helsinki Declaration as revised in 2013
COPE flowcharts offer a step-by-step process, for practical use on handling different aspects of publication ethics issues. https://publicationethics.org/node/19631
Submission Process:
All manuscripts of JLMDC should be submitted through the online submission system through the journal website. The corresponding/principal author is responsible for the manuscript during the submission and the entire peer-review process. The corresponding author must ensure that all other authors of the manuscript have read and approved the submitted version of the manuscript. For submission of the manuscript, the author must Register and Login into the OJS and submit the following along with the manuscript according to the guidelines mentioned below.
Ethical Approval letter: Letter of approval from the relevant ethical review board, along with its reference number.
Letter of undertaking and submission statement: JLMDC Undertaking .pdf
Cover Letter: Cover letter pdf
Title Page: The complete title of the manuscript, running title (40 characters), the name of all the authors with qualifications, the affiliated department or institution, address for correspondence with telephone numbers, cell phones, email, and a short running title of the article, source of funding, and total word count.
Specimen for title page available at: Guidelines for Title Page
Abstract:
Introduction: This manuscript part should be composed of a deductive approach. This should specify the background and the rationale for the study, with reference to national, regional and international literature. It should also briefly describe the purpose of the study. By the end of the introduction, the author should provide a road map that outlines your main points.
Methodology: This section should include Study design, Settings and Study duration. Sampling method and sample size calculations should be mentioned and the following to be included as subheadings in an organized manner. Ethical consideration: (Ethical review statement, confirming that informed written consent was obtained from all study participants, ensuring confidentiality of their information, must be included along with ethical approval letter Number and date) Inclusion Criteria, Exclusion Criteria and Statistical Analysis. How the data was collected or generated, details of apparatus (if applicable with manufacturer's name and address) and or drugs/chemicals used. Use generic name/s of drugs/ chemicals along with dose (s) and route(s) of administration. Statistical analysis should include the tests applied along with the statistical software package used with the version. For patients, age, and sex with mean age ±standard deviation must be given. Please specify any general computer program used.
Results: The results should justify the claims. Authors should summarize the results through text, graphs, tables and figures, to report the statistical findings. Repetitions of data should be avoided in tables and figures if already described in the text.
Discussion: Authors should describe and discuss the significance of the study findings in light of what was already known about the investigated research problem. Explain any new findings or fresh insights about the problem. Strengths and limitations should be included at the end of this section.
Conclusion(s): Authors should briefly summarize the net findings of the study without overemphasizing them. It should be in line with the results.
Disclosure:
When authors incorporate Artificial Intelligence or AI-assisted tools in drafting their manuscripts, their use should be limited to enhancing language and readability. These technologies should not replace essential research tasks such as data interpretation or drawing scientific conclusions. It is crucial that AI is employed under human supervision and control, with careful review and editing of the output, as AI-generated content may appear credible yet still contain errors, omissions, or biases.
AI or AI-assisted tools cannot be credited as an author or co-author, nor should they be cited as an author. Authorship entails specific responsibilities that only humans can fulfill. Authors must disclose any use of AI in manuscript writing and preparation. If AI has been utilized, a statement reflecting this will be included in the published work. Nonetheless, the authors remain fully responsible and accountable for the content of their manuscript.
Authors Contribution Statement: All the authors contributed equally in accordance with ICMJE guidelines and are accountable for the integrity of the study.
Authorship Criteria Recommended by ICMJE:
The ICMJE recommends that authorship be based on the following criteria:
Ethical consideration (Letter No): A statement confirming that informed written consent was obtained from all study participants, ensuring confidentiality of their information, must be included. Additionally, the letter of approval from the relevant ethical review board, along with its reference number, should be provided.
Acknowledgment: Names and contributions of people who helped in the study, but do not qualify as authors may be listed in this section with their permission.
Tables and Figures: These must be incorporated into the manuscript in accordance with specified requirements. They should be clear and non-redundant, avoiding repetition of already-discussed results or data in the text. Tables should be sequentially numbered in Roman numerals, featuring concise and explanatory titles at the top. Abbreviations, if employed, must be explained in a footnote. Statistical results should encompass both standard deviation and standard error of the mean. Additionally, proper attribution to published articles is required for any data or tables sourced from them. The maximum number of tables/ figures in original articles should be 6, for review articles should be 6, for case reports should be 6, and for short communication should be 2. Refer to the table number in the relevant section of your manuscript. Additional 1000 PKR./- (USD 10) will be charged for each figure & table if exceeds the maximum number.
Units of Measurement: For reporting in the text, they should be conventional with System International (SI) units given in parenthesis. The names of drugs should be written in generic terminology.
References: All references should be cited in Vancouver style and give citations in the text as superscript. While citing articles use references of published articles and avoid personal communications and unpublished observations. These references should also be marked in the text. To minimize errors, the author should verify references against the original documents. The references should be written in Vancouver style as per "Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in medical journals, updated in December 2015. Available at: https://icmje.org/recommendations. List all authors when there are six or fewer. If there are seven or more, list the first six followed by” et al” reference should provide the following information: Author’s name with initials, the full title of cited article, name of the journal in which the article appeared (in Abbreviation), Year of Publication, journal’s volume number, and finally first &; the last page numbers. Write page no. like this 120-126. Add DOI number of those references where it is available.
Examples
Reference from the original article: Denny KJ, Woodruff TM, Taylor SM, Callaway LK. Complement in pregnancy: a delicate balance. Am J Reprod Immunol. 2013;69(1):3-11. doi: 10.1111/aji.12000.
Reference from books: Stronge JH. Qualities of effective teachers. 3rd ed. Ascd. 2018. [https://www.middleweb.com/38903/reflect-on-the-qualities-of-effective-teachers]
Reference from the website (Other than the original article): WHO. WHO recommendation on calcium supplementation before pregnancy for the prevention of pre-eclampsia and its complications. 2022 Apr 17 [Cited 2022 Oct 19]. Available from: https://www.who.int/publications/i/item/9789240003118https://www.who.int/publications/i/item/9789240003118
Word limits and number of references: Word limit for submission of each manuscript is as under excluding abstract & references. The maximum length of the manuscripts should be: Original Articles: 3500 words and, in exceptional cases 4000, Case Reports/Brief Communications/Mini Review: 2000 words, Review Articles: 4500 words including summary describing the methods used for locating, selecting, extracting and synthesizing data. Maximum References: Original Article 25, Mini Review 25, Case Report 12, Short Communication 20, Reviews 40. These references should also be marked in the text.
Case Reports: These consists of unique cases, either diagnosed or reporting of treatment, in general, all case reports include the following components: an abstract, an introduction, a case, and a discussion. Have an unstructured abstract of approximately 150 words, up to 12 references, six tables and figures. Figures should be submitted as sperate files. Consent needs to be obtained prior to taking any patient’s photographs.
Abstract: A non-structured abstract should be written as a case statement for a case report with minimum of three keywords.
Introduction: Provided a clear statement of the problem, the relevant literature on the subject,
Case Report: This section should provide the details of the case in the following order: Patient description, Case history, Physical examination results, Results of pathological tests and other investigations, Treatment plan, Expected outcome of the treatment plan, Actual outcome.
The author should ensure that all the relevant details are included, and unnecessary ones excluded.
Discussion: Describes detailed interpretation of data. Pertinent literature support is provided for any statement, and no assumption is made.
Table/Figures: Either three concise tables or three figures. Figure legends should be typed in numerical order.
References: References are listed at the end of the paper in numerical order. Journal names are according to Vancouver style. The number of references for the Case Report should be 12. Preferably, fifty percent references should be from the last five years.
Review Article
Overview and analysis of the topic with background and latest updates must be addressed in all kind of reviews i.e. systematic review, narrative review and evidence based review in line with the original article. It should include original work of author on the similar subject. The suggested length for review article is 2500 to 3000 words with 40-50 references. The abstract should be non-structured with 150 words and at least 3 keywords.
Letter to the Editor
The letter to the editor should be Objective, constructive and purposeful. It should offer Novel or useful information and be worth publishing, either in addition to or in lieu of on previous opinions or experience to previous published articles. Letters should be short and be concise, with clear and specific points. It may not often exceed 500 words and 5-7 references.
Guest Editorials
Guest Editorials should comprise 600-700 words (maximum 1000) with 5-8 references. There can be maximum 3 authors. It is mandatory to state acknowledgment and funding disclosure if any.
Policy of Screening Plagiarism: JLMDC follows the standard definition and description of plagiarism and we endorse The COPE, ICMJE, & Higher Education Commission (HEC) Pakistan policies regarding plagiarism. JLMDC is equipped with Turnitin (text-matching/anti-plagiarism software provided by the Higher Education Commission of Pakistan). This is used to check for plagiarism and redundancy in submitted manuscripts. All submitted manuscripts are first checked for similarity index on Turnitin.
A manuscript derived from an author's M.Phil. or Ph.D. thesis must explicitly state the "title of the thesis," "Supervisor name," Department and University name, and "year of submission" on the title page as part of the submission process.
Note: Authors must not check plagiarism through unreliable or unauthentic websites or softwares.
Processing and Publication Charges: All manuscripts from Pakistan should be accompanied with a payment of Rs. 7,000/- FOR overseas (US$ 55)/- (Non-refundable). APC charges, once submitted, are non-refundable. Once the manuscript is accepted for publication, the authors are required to pay publication charges of Rs.18,000/- For Overseas US$ 200/- in the name of (Journal of Lahore Medical and Dental College), Lahore; send us on the address given below.
Prof Dr. Zaima Ali
Editor in Chief, JLMDC
Lahore Medical & Dental College, Lahore, Pakistan.
Email: editorjlmdc@lmdc.edu.pk .
Website: https://jlmdc.lmdc.edu.pk/index.php/lmdc
Note:
The Journal has adopted a full fee waiver policy to support authors and encourage submissions (until recognition by any of the two regulatory bodies i.e. PMDC and HEC).
Processing and Publication charges from authors are taken to ensure their Open Access availability for the readers.
Bank Name: Bank Alfalah
Account Title: Lahore Medical and Dental College
Account Number: 1009598114
IBAN Number: PK31ALFH0812001009598114
Editing Charges (Optional) Rs. 7000/- For overseas (US$ 100)
It will cover the following aspects of the manuscript
Printed Copy: One printed copy will be supplied to the correspondence author. Authors can order additional copies at the rate of Rs. 1000/- per copy which includes courier charges and US$ 20/- per copy for overseas. Payment for additional copies should be added in the publication charges.
Annual Subscription Rates for Print Copy
|
Annual Subscription: |
Rs. 2500/- |
|
3 Year’s Subscription: |
Rs. 5000/- |
|
5-Year’s Subscription: |
Rs. 8000/- |
Fast Track Processing of Manuscript: The duration of the fast-track processing is approximately 40 days. If the author wants to get his/her manuscript through fast-track, He/she should clearly mention it on the cover letter. Rs. 10,000 additional fee will be charged (other than the processing fee of Rs. 7000/- US$ 50 for overseas) at the time of submission (Due to the increasing cost of publication, the management has imposed a fast-track fee of Rs. 10,000/- US$ 200 for overseas). The fast-track charges are non-refundable. The rest of the terms and conditions will remain constant. There is no guarantee of acceptance or publication in fast-track. Authors are requested not to opt for fast-track processing unless it is extremely essential.
Waiver Policy:
Editor In Chief is responsible for the final decision.
Eligibility criteria to apply for waiver:
Editorial Processing:
 Peer Review Policy:
Peer Review Policy:
Once a manuscript clears the initial screening, it is sent for peer review.
The peer review is completed once all the reviewers send a detailed report to the journal with their comments on the manuscript and their recommendations. We request the reviewers to complete their reviews within 2-4 weeks.
Final decision: The journal editor or editorial board considers the feedback provided by the peer reviewers and arrives at a decision. The following are the most common decisions that are made:
Deadline of Process
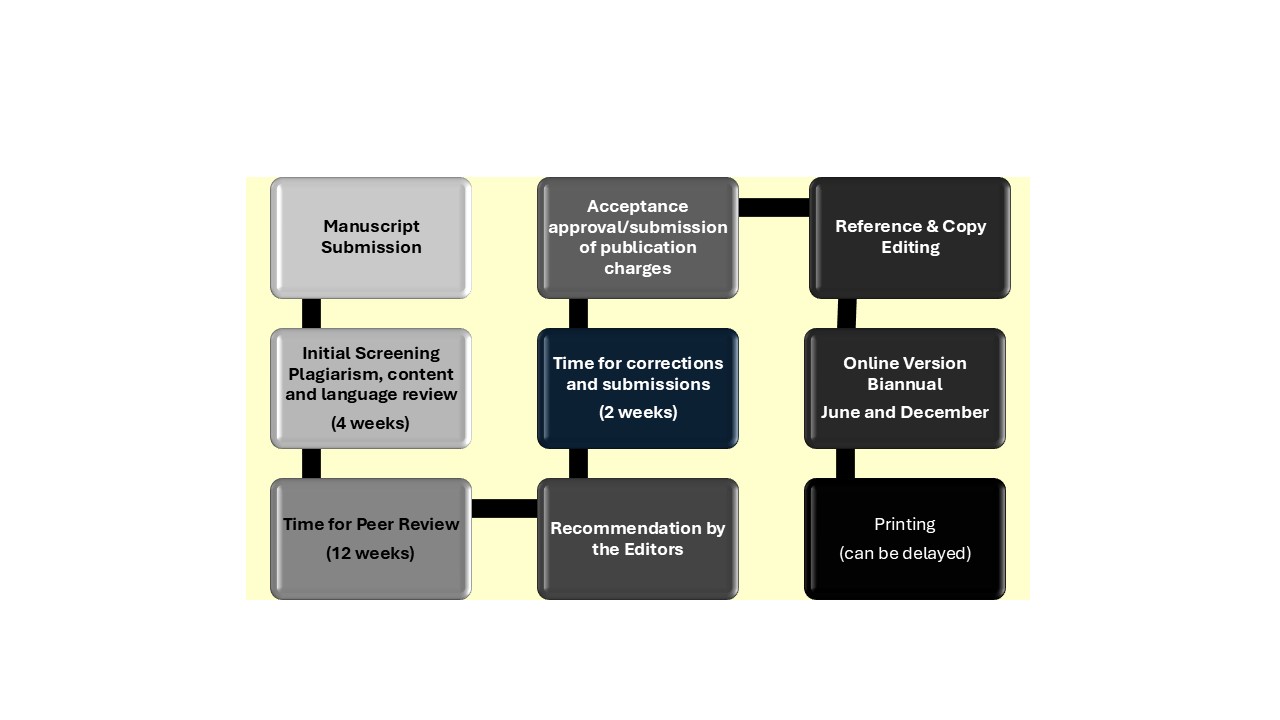
Publishing Schedule:
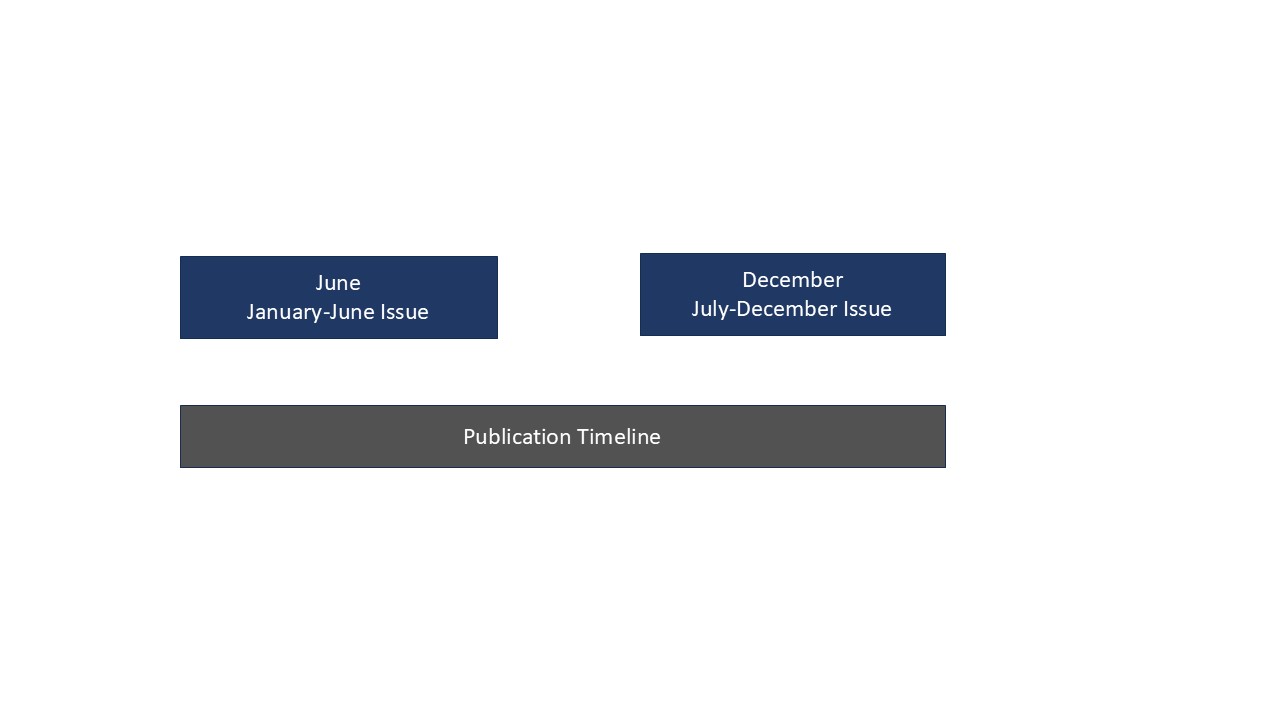
Original research articles, review articles, practical updates, case reports, and letters to the editor of medical and allied health sciences are being evaluated by the editorial board and peer reviewers before publication in the journal. Under the following terms:
![]()
Sponsers:
Lahore Medical & Dental College, Lahore, Pakistan.
Journal of Lahore Medical & Dental College is an open-access journal being published since January 2024. The journal only publishes open access content and legacy. These articles are published in open-access under CC BY (Creative Commons Attribution 4.0 International License). The CC BY license allows for maximum dissemination and re-use of open access materials and is preferred by many research funding bodies. Under this license users are free to share (copy, distribute and transmit) and remix (adapt) the contribution, including for commercial purposes, providing they attribute the contribution in the manner specified by the author or licensor.
Under Creative Commons, authors retain copyright in their articles.
Visit our open research site for more information about Creative Commons licensing.
Benefits of open access:
Open access publications lead to:
Ethical Approval of the Research: Ethical approval from the organization overseeing the study participants is mandatory.
Examples of research which has implications requiring ethical approval include in particular:
Strict compliance to ethical standards should be observed while dealing with subjects. Studies on human subjects must indicate explicitly that the procedures followed were in line with the revised “Helsinki Declaration” of 1983. Detailed guidelines are available from the following link: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct1983. Manuscripts of animal studies should clearly indicate that WMA Statement on Animal Use in Biomedical Research were followed. The guidelines on animal research ethics are available from; https://www.wma.net/policies-post/wma-statement-on-animal-use-in-biomedical-research/ to safeguard the rights and welfare of human subjects participating in biomedical research, ethical approval must be obtained for all manuscripts from the Hospital/ Institutional Ethical Committee(s) of the concerned organization(s). All the ethical approval(s), in the case of multicenter studies, should accompany the manuscript in writing. Conflict of interest any conflict of interest should be declared. It may include honorarium, funding, credits, and promotion which are not unethical but should be declared.
Guidelines for Research Involving Vulnerable Populations:
Before enrolling any vulnerable participant, it is crucial to obtain explicit informed consent. For minors, consent must be obtained from their legal guardians. Moreover, seeking assent from minors, taking into account their age and maturity, should be pursued whenever feasible.
The process of securing informed consent should entail a thorough disclosure of information regarding the study's purpose, goals, potential risks, benefits, and alternative options for participation. This information should be communicated clearly and understandably, tailored to the participants' level of comprehension.
Preserving the autonomy and decision-making capacity of participants is paramount, ensuring their unfettered right to withdraw from the study at any point without facing adverse consequences.
For vulnerable populations lacking decision-making capacity, such as individuals with severe cognitive impairments, consent should be obtained from their legally authorized representatives or next of kin. This ensures decisions are made in the best interest of the participant.
All research protocols involving vulnerable populations must undergo thorough scrutiny and approval by an independent ethics committee or institutional review board (IRB). These review bodies should include members with expertise in the specific vulnerabilities of the population being studied.
Researchers have a duty to diligently mitigate potential risks and discomforts for vulnerable participants. Rigorous risk-benefit analyses should be conducted to ensure that the potential benefits of the research outweigh any potential risks significantly.
Continuous monitoring and assessment of participants' well-being throughout the research process are paramount. Robust measures should be in place to promptly address any unforeseen adverse effects that may arise.
Clinical Trials with Vulnerable Populations:
Appeal Process: JLMDC depends on a proficient editorial board to render editorial decisions and offer feedback to authors. Should you wish to contest the rejection of a submitted article to JLMDC, kindly reach out to the Editor-in-Chief of the journal.
Self-Archiving Policy of JLMDC:
a: Preprint: A preprint is described as the author’s version of the manuscript before peer-review. Journal of Lahore Medical & Dental College neither accepts nor publishes pre-prints.
b: Author’s Accepted Manuscript before Publication (AAM): There is an embargo period on all submitted manuscripts till the time of publication on JLMDC website. This embargo period does not apply on abstracts.
C: Published Article (Version of Record): The version of the manuscript that is published (online/print) in a journal following peer review, copyediting and typesetting.
Complaint Process: The Journal of Lahore Medical & Dental College prioritizes transparency at all stages. Authors retain the right to raise concerns and seek clarification regarding any perceived ethical misconduct or policy breaches. JLMDC will promptly acknowledge receipt of any email correspondence sent to complaintjlmdc@lmdc.edu.pk with in 2 weeks. The decision is taken in and the same is forwarded to the concerned scholar through his submitted email ID. If the author wishes to pursue their complaint further, they may contact COPE directly. Information can be found on the COPE website: Facilitation and Integrity Subcommittee | COPE: Committee on Publication Ethics.
Complaints may pertain to undisclosed conflicts of interest, plagiarism, multiple or duplicate publications, simultaneous submissions, unethical research practices, falsification of results, breaches of research standards, reviewer bias, or any contributions to JLMDC that infringe upon copyright or other intellectual property rights.
Advertisement Policy: We accept advertisements from pharmaceutical companies for our print editions. The decision to accept these advertisements lies with the Editor-in-Chief. They are displayed randomly throughout the publication. For advertising inquiries, please contact: editorjlmdc@lmdc.edu.pk .
Withdrawal Policies: According to JLMDC’s copyright policy, the copyright belongs to JLMDC submission. Furthermore, it is considered unethical to submit the same manuscript to more than one journal at the same time. Withdrawal of the manuscript during its editorial workflow has been divided into parts.
Refund Policy: The author bears the responsibility for payment, and refunds will not be provided. Once the payment for APC/Fast Track is confirmed and processed, no refunds will be issued. Should you encounter any difficulties with online payment, we advise reaching out to us for assistance. It's important to note that the APC for each paper is applicable only once and is non-refundable. In the event of paper withdrawal from the journal, APC will not be reimbursed to the author.



Print ISSN: 3007-9462
eISSN:3007-8393
J Lah Med Dent Coll is the Journal of Lahore Medical & Dental College.
Journal of Lahore Medical and Dental College © 2024 sponsored by Lahore Medical and Dental College is licensed under CC BY 4.0 .
